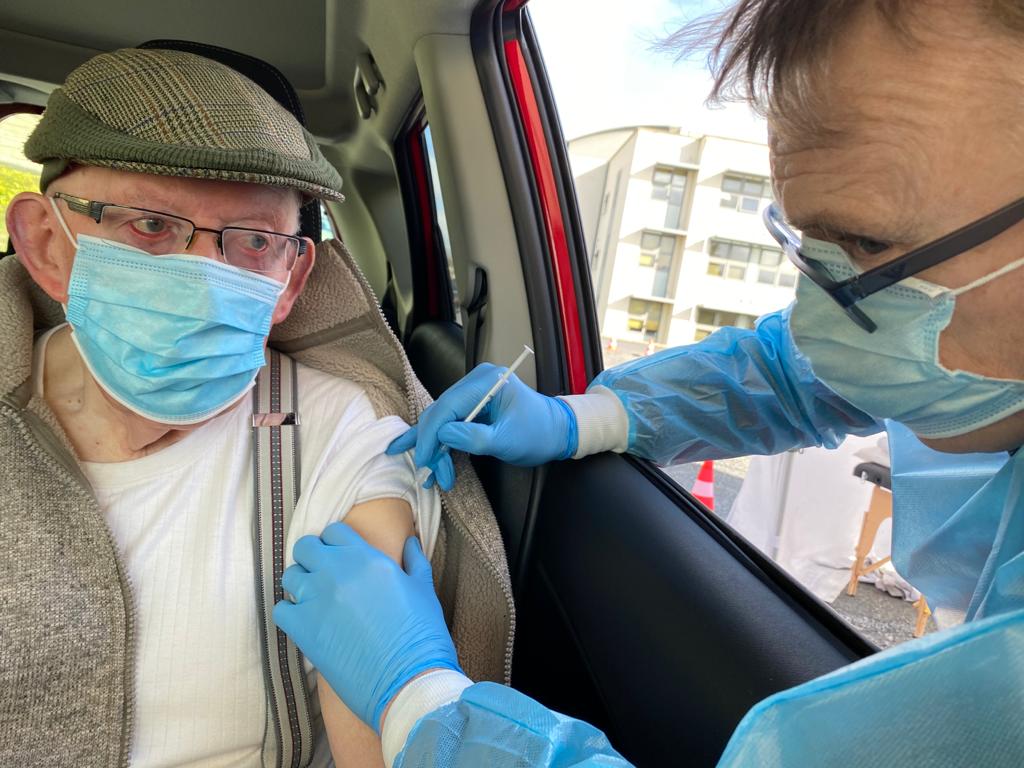
Europe’s health regulator is evaluating an application for the use of a booster dose of the Pfizer Covid-19 vaccine.
It would involve administering a dose of the vaccine 6 months after the second dose in people aged 16 and over.
A European Medicines Agency committee will carry out an accelerated assessment of data submitted by the company, and the outcome is expected in the next few weeks.
The Irish government is already considering the use of a booster shot for older and medically vulnerable people ahead of the winter, possibly in conjunction with a flu jab.