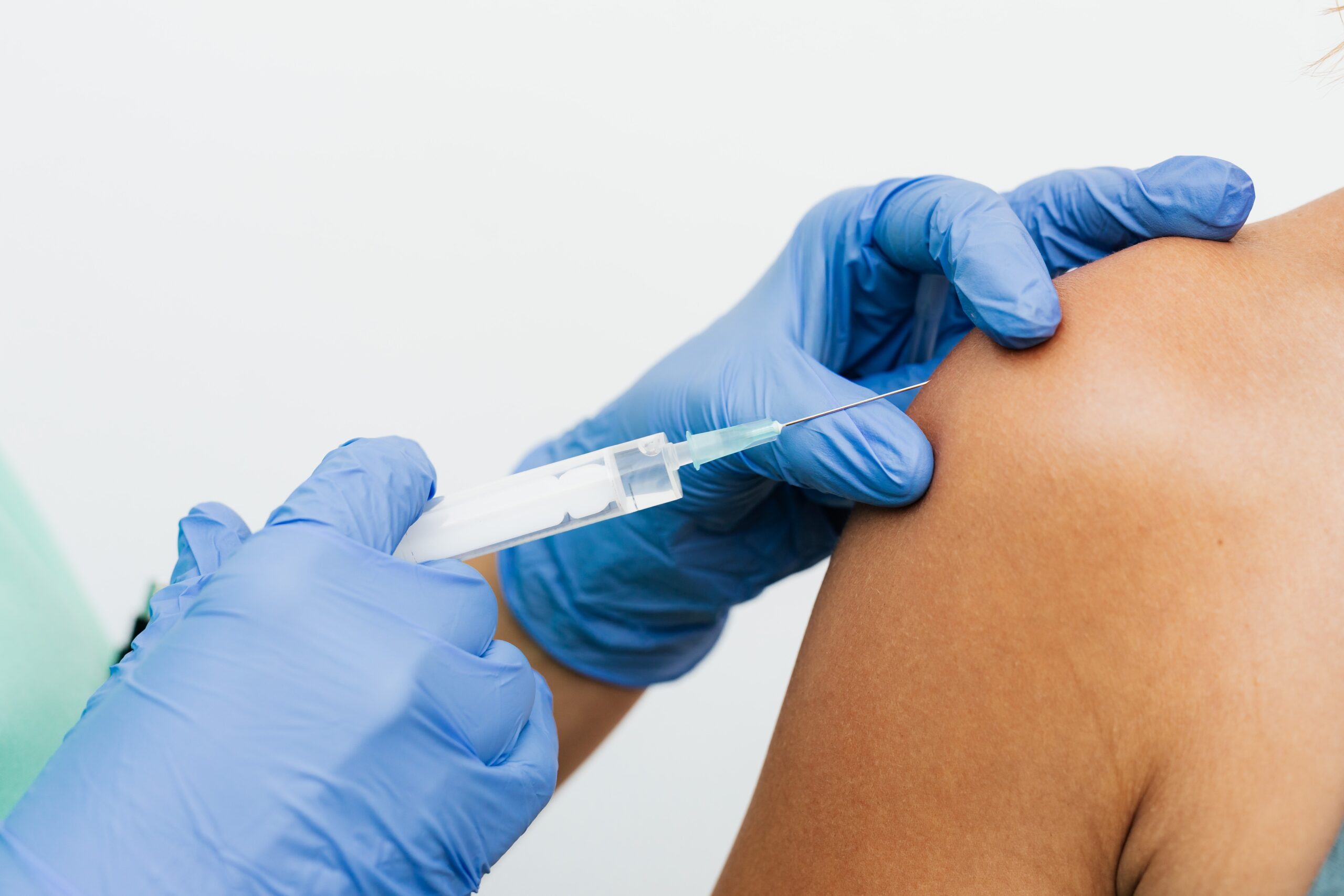
The EMA has approved the Moderna vaccine.
It follows a meeting this morning by the regulatory body and is the second vaccine approved by for use throughout the European Union.
The European Medicines Agency granted conditional marketing authorisation for the vaccine to combat the spread of Covid-19.
It’s a two-dose vaccine, with injections given 28 days apart, and follows the approval by the body last month on the Pfizer jab.
The EMA’s Emer Cooke said: “This vaccine provides us with another tool to overcome the current emergency.
President of the European Commission Ursula von der Leyen says it is good news and the commission is working at full speed to approve it and make it available throughout the EU.
Ireland has ordered 850,000 doses.